Top Stories
FDA Issues Urgent Botox Warning After Serious Illness Reports
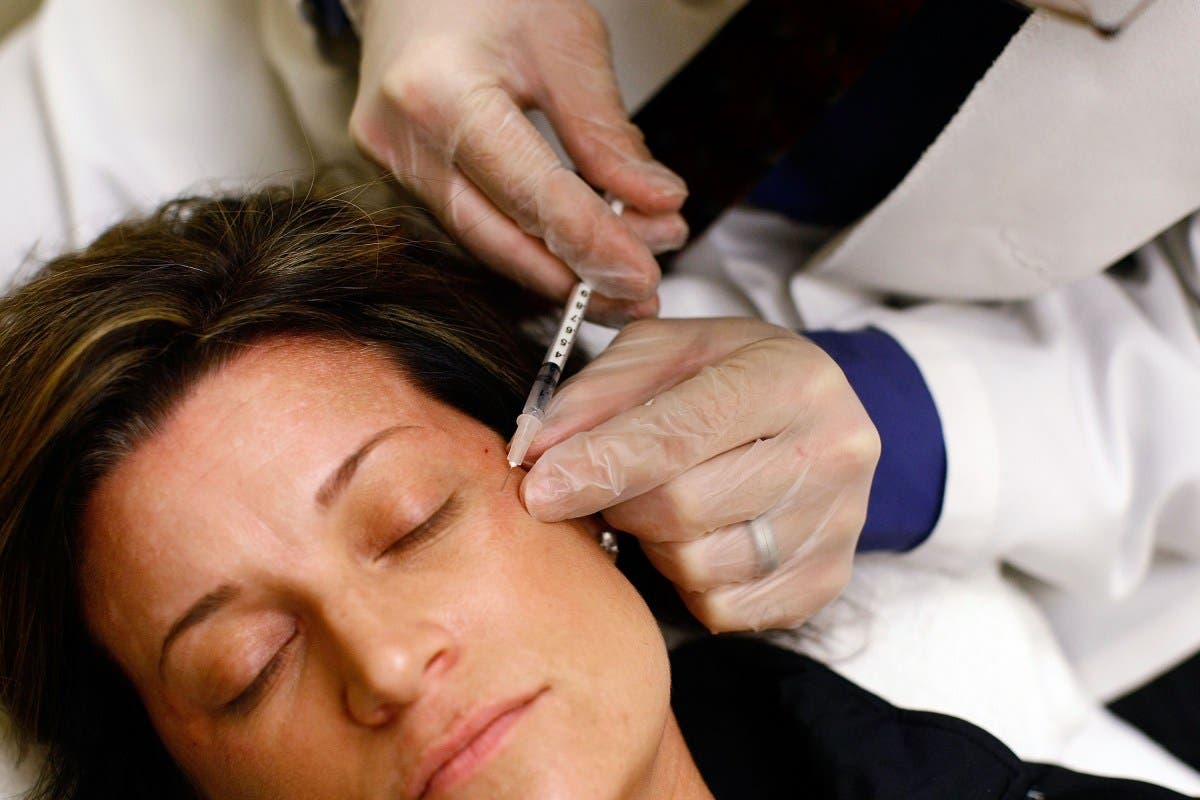
UPDATE: The U.S. Food and Drug Administration (FDA) has issued an urgent warning regarding unapproved Botox products following reports of a rare but serious illness linked to botulism. This announcement comes as officials report an alarming increase in adverse effects associated with unauthorized injections.
The FDA has sent warning letters to 18 websites accused of illegally marketing counterfeit Botox products. These actions follow confirmed cases of botulism, a potentially fatal condition caused when the toxin spreads beyond the injection site. Symptoms can include muscle weakness, difficulty swallowing or breathing, and, in severe cases, death.
Why This Matters: Botulinum toxin, commonly known as Botox, is widely used for cosmetic purposes, particularly to reduce wrinkles by paralyzing facial muscles. While FDA-approved Botox is considered safe when administered by licensed professionals, unapproved or counterfeit injections pose significant health risks.
From November 2023 to March 2024, the Centers for Disease Control and Prevention (CDC) documented incidents involving at least 22 individuals across 11 states who suffered adverse reactions after receiving suspicious injections, frequently performed outside licensed healthcare settings. Most affected individuals were women aged 25 to 59. Reports included symptoms ranging from drooping eyelids to severe respiratory distress, with 11 individuals requiring hospitalization.
The FDA’s enforcement action is part of a broader effort to combat the sale of unauthorized and misbranded products online. Websites targeted include acecosm.com, aesthetic-essentials.com, and several others. FDA Commissioner Marty Makary emphasized the seriousness of the issue, stating,
“Unapproved and misbranded Botox products carry serious health risks. Today we’re taking action to protect American consumers and prevent online entities from selling these dangerous products.”
Dr. Oma Agbai, a board-certified dermatologist, urged patients to advocate for their own safety when considering cosmetic procedures. “Patients should take an active role in advocating for their own safety,” she stated.
What to Do: The FDA advises consumers to verify both the product and the provider before receiving any botulinum toxin injection. If you have received a suspicious injection and experience symptoms such as vision changes, muscle weakness, or difficulty breathing, seek immediate medical attention.
Next Steps: The FDA, CDC, and state health departments are closely monitoring adverse event reports linked to unauthorized botulinum toxin products. They urge anyone affected to report their experiences and seek medical care promptly. As this situation develops, the health and safety of consumers remain at the forefront of regulatory efforts.
Stay informed as this story unfolds and ensure your cosmetic procedures are performed by qualified professionals using approved products.
-

 Science2 weeks ago
Science2 weeks agoInventor Achieves Breakthrough with 2 Billion FPS Laser Video
-

 Top Stories3 weeks ago
Top Stories3 weeks agoCharlie Sheen’s New Romance: ‘Glowing’ with Younger Partner
-

 Entertainment3 weeks ago
Entertainment3 weeks agoDua Lipa Aces GCSE Spanish, Sparks Super Bowl Buzz with Fans
-

 Business3 weeks ago
Business3 weeks agoTyler Technologies Set to Reveal Q3 Earnings on October 22
-

 Health3 weeks ago
Health3 weeks agoCommunity Unites for 7th Annual Into the Light Walk for Mental Health
-

 Health3 weeks ago
Health3 weeks agoCurium Group, PeptiDream, and PDRadiopharma Launch Key Cancer Trial
-

 World3 weeks ago
World3 weeks agoR&B Icon D’Angelo Dies at 51, Leaving Lasting Legacy
-

 Entertainment3 weeks ago
Entertainment3 weeks agoRed Sox’s Bregman to Become Free Agent; Tigers Commit to Skubal
-

 Entertainment3 weeks ago
Entertainment3 weeks agoMother Fights to Reunite with Children After Kidnapping in New Drama
-

 Health3 weeks ago
Health3 weeks agoNorth Carolina’s Biotech Boom: Billions in New Investments
-

 Science3 weeks ago
Science3 weeks agoNorth Carolina’s Biotech Boom: Billions Invested in Manufacturing
-

 Top Stories3 weeks ago
Top Stories3 weeks agoDisney+ Launches Chilling Classic ‘Something Wicked’ Just in Time for October