Business
Revolutionary ‘Butt Breathing’ Technique Advances in Human Trials
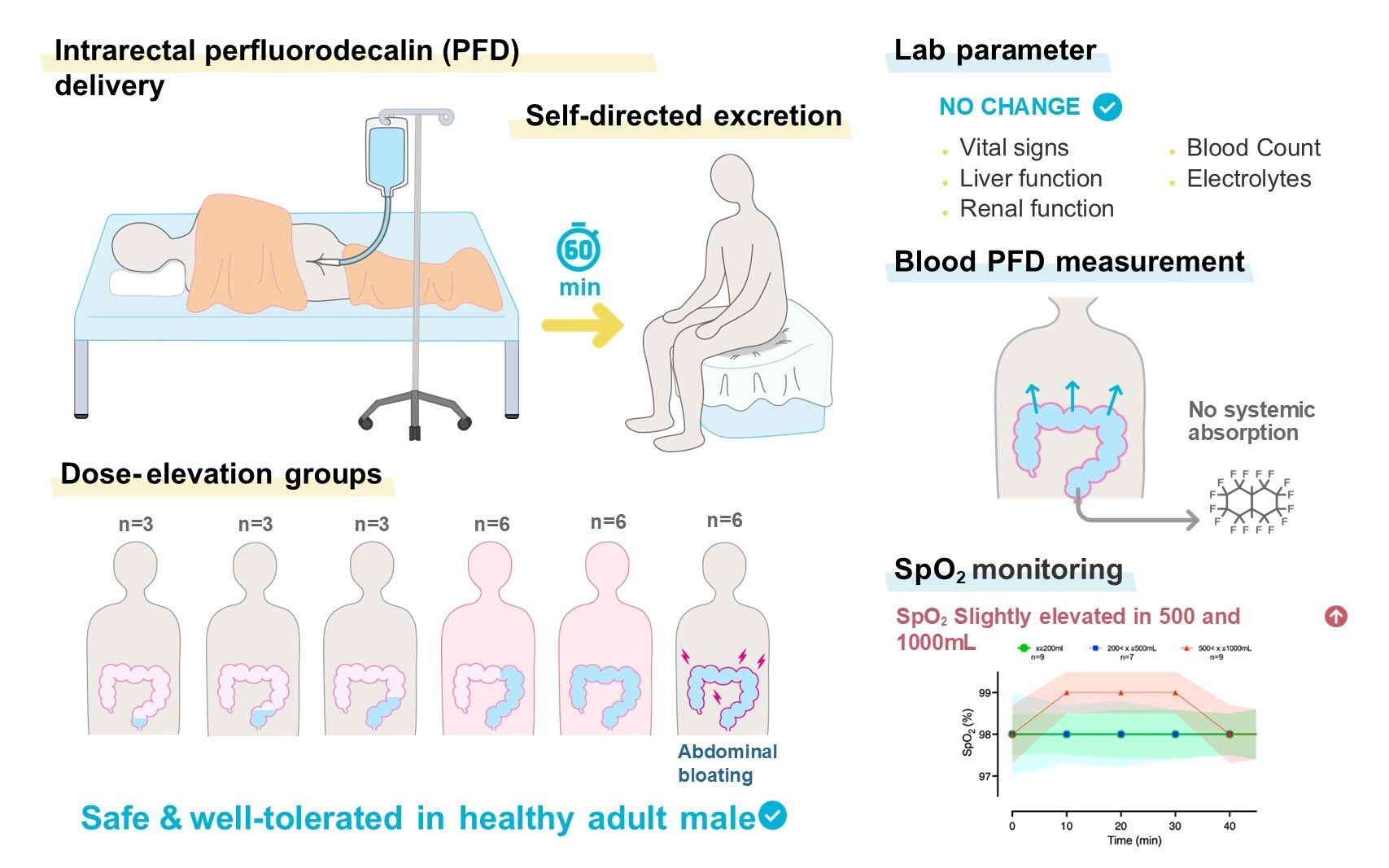
A groundbreaking medical concept known as “enteral ventilation,” which involves delivering oxygen rectally to assist individuals with blocked airways, has achieved a significant milestone in human testing. On October 20, 2025, results from the first clinical trial were published in the journal Med, indicating that the technique, initially awarded an IgNobel Prize in 2024, demonstrates safety in human subjects.
Dr. Takanori Takebe, a prominent researcher in organoid medicine at Cincinnati Children’s Hospital Medical Center and the University of Osaka, led the study. He emphasized that while the initial findings confirm the procedure’s safety, they do not yet establish its effectiveness. “This is the first human data, and the results are limited solely to demonstrating the safety of the procedure and not its effectiveness,” Takebe explained. “Now that we have established tolerance, the next step will be to evaluate how effective the process is for delivering oxygen to the bloodstream.”
Understanding Enteral Ventilation
The concept of enteral ventilation involves an enema-like technique where a highly oxygenated liquid is introduced into the rectum, allowing oxygen absorption through the colon into the bloodstream. This innovative approach could provide vital support for patients with compromised lung function due to conditions such as severe infections or injuries that obstruct airways.
The initial groundwork for this technique was laid by observing the loach, a type of fish capable of absorbing oxygen through its gut under low-oxygen conditions. Additionally, it draws upon the earlier work of Leland Clark, a former researcher at Cincinnati Children’s, who created a perfluorocarbon liquid known as Oxycyte. Although Oxycyte did not progress as an artificial blood substitute, its concept gained cultural recognition through its portrayal in the 1989 film “The Abyss.”
Trial Findings and Future Steps
The recent study involved 27 healthy men in Japan who were instructed to retain varying amounts of the perfluorocarbon liquid for up to 60 minutes. Notably, 20 participants managed to hold the liquid for the entire duration, with volumes reaching up to 1,500 ml. While some participants reported mild abdominal bloating and discomfort, there were no serious adverse events recorded.
The next phase of research will focus on using oxygenated versions of the liquid to determine the optimal volume and duration needed to enhance blood oxygen levels effectively. There is also an aspiration to adapt this technology for use in neonatal care. To further this project, Takebe has established a company named EVA Therapeutics, which aims to advance the development of enteral ventilation.
Funding will be a crucial factor in the timeline for future clinical trials, as Takebe noted that the pace of fundraising will dictate the progress of this innovative medical approach. As the research continues, the potential for enteral ventilation to transform emergency care for patients with respiratory distress remains a captivating prospect in modern medicine.
-

 Science4 months ago
Science4 months agoInventor Achieves Breakthrough with 2 Billion FPS Laser Video
-

 Science2 months ago
Science2 months agoResearchers Launch $1.25M Project for Real-Time Hazard Monitoring in Hawaiʻi
-

 Top Stories4 months ago
Top Stories4 months agoCharlie Sheen’s New Romance: ‘Glowing’ with Younger Partner
-

 Entertainment4 months ago
Entertainment4 months agoDua Lipa Aces GCSE Spanish, Sparks Super Bowl Buzz with Fans
-

 World4 months ago
World4 months agoUK Government Borrowing Hits £20.2 Billion in September Surge
-

 Health4 months ago
Health4 months agoCommunity Unites for 7th Annual Into the Light Walk for Mental Health
-

 Entertainment4 months ago
Entertainment4 months agoMother Fights to Reunite with Children After Kidnapping in New Drama
-

 Entertainment4 months ago
Entertainment4 months agoOlivia Plath Opens Up About Her Marriage Struggles and Divorce
-

 Science3 months ago
Science3 months agoAI Gun Detection System Mistakes Doritos for Weapon, Sparks Outrage
-

 Top Stories4 months ago
Top Stories4 months agoFormer Mozilla CMO Launches AI-Driven Cannabis Cocktail Brand Fast
-

 Health4 months ago
Health4 months agoYouTube Launches New Mental Health Tools for Teen Users
-

 World4 months ago
World4 months agoR&B Icon D’Angelo Dies at 51, Leaving Lasting Legacy









