Health
New Vanderbilt Technique Uncovers Genes Linked to Schizophrenia Risk

A groundbreaking technique developed at Vanderbilt University has identified new risk genes associated with schizophrenia, a complex psychiatric disorder affecting approximately 1 percent of the population, or around 3.5 million people in the United States. This advancement holds significant potential for future drug development, aiming to address the underlying biological mechanisms of the disease rather than merely its symptoms.
Schizophrenia is recognized for its strong genetic component, with an estimated heritability of around 80 percent. Traditional genetic research has primarily focused on identifying genes with the most substantial impact on risk. To date, more than 200 loci have been linked to schizophrenia through a genetic research method known as Genome-Wide Association Studies (GWAS). However, researchers believe that over 1,000 genes may play a role in the disorder, indicating a significant number of risk-related genes remain undiscovered.
Innovative Approach to Gene Discovery
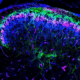
In a recent study published in Advanced Science, the lab led by Bingshan Li, a professor of molecular physiology and biophysics, expanded the traditional parameters for identifying risk genes. The research focused on “weaker” genetic signals that had previously been overlooked. By examining these signals, the team discovered that dysfunctions in two critical biological processes, specifically the development and morphology of dendrites—neuron projections vital for brain communication—are implicated in schizophrenia.
Rui Chen, a research instructor at Vanderbilt and one of the study’s co-first authors, explained, “Even though large genetic studies show that schizophrenia is strongly influenced by DNA, the usual analyses miss many weaker—but meaningful—signals. Our study looks beyond the standard cut-off to include these ‘near-miss’ genetic clues, finding issues in how neurons grow and shape their dendrites.”
The research team also included co-first authors Quan Wang and Benjamin Siciliano from Zhexing Wen’s lab at Emory University. They utilized human induced pluripotent stem cell-derived neurons to confirm their findings, revealing that higher expression levels of two implicated genes disrupted normal dendrite growth. This discovery highlights a clear biological pathway connecting genetic risk to the underlying mechanisms of schizophrenia.
Key Findings and Future Directions
The study yielded three key findings that could significantly influence future research and treatment strategies:
1. **Hidden Genetic Clues Matter:** The investigation revealed that many “near-miss” genetic changes contain essential information about schizophrenia risk, which traditional analyses often overlook.
2. **Neuron Dendrite Morphogenesis is Key:** The identified hidden genetic signals pointed to a pathway governing how brain cells grow and branch out their connections. Disrupted dendrite morphogenesis may be a critical factor in schizophrenia.
3. **iPSC Modeling Confirms Abnormal Dendritic Morphogenesis:** Testing two near-miss genes, DCC and CUL7, in human induced pluripotent stem cell-derived neurons demonstrated that increasing their expression resulted in shorter dendrites with fewer branches, directly linking genetic risk to structural changes in the brain.
Chen expressed hope that the findings will shift the perception of schizophrenia from merely a “chemical imbalance” to a disorder involving how brain cells connect. This perspective could guide future studies, inspire new experimental models, and pave the way for identifying drug targets aimed at restoring healthy brain cell connections.
The research team aims to extend their work by utilizing larger genetic datasets to uncover additional schizophrenia risk genes and pathways. They plan to explore how these risk genes affect brain cell connections throughout development, with the goal of identifying new therapeutic targets that could alter the disease’s trajectory.
The study represents a collaborative effort among experts in genetics, molecular biology, and neuroscience, highlighting the strengths of Vanderbilt University. The unique environment fostered comprehensive teamwork and innovation, essential for advancing understanding in schizophrenia genetics.
This research received funding from the National Human Genome Research Institute, National Institute of Mental Health, and the National Institute on Aging, reflecting its importance in the ongoing battle against mental health disorders.
-

 Science4 weeks ago
Science4 weeks agoInventor Achieves Breakthrough with 2 Billion FPS Laser Video
-

 Health4 weeks ago
Health4 weeks agoCommunity Unites for 7th Annual Into the Light Walk for Mental Health
-

 Top Stories4 weeks ago
Top Stories4 weeks agoCharlie Sheen’s New Romance: ‘Glowing’ with Younger Partner
-

 Entertainment4 weeks ago
Entertainment4 weeks agoDua Lipa Aces GCSE Spanish, Sparks Super Bowl Buzz with Fans
-

 Business4 weeks ago
Business4 weeks agoTyler Technologies Set to Reveal Q3 Earnings on October 22
-

 Entertainment4 weeks ago
Entertainment4 weeks agoMother Fights to Reunite with Children After Kidnapping in New Drama
-

 Health4 weeks ago
Health4 weeks agoCurium Group, PeptiDream, and PDRadiopharma Launch Key Cancer Trial
-

 World4 weeks ago
World4 weeks agoR&B Icon D’Angelo Dies at 51, Leaving Lasting Legacy
-

 Health4 weeks ago
Health4 weeks agoNorth Carolina’s Biotech Boom: Billions in New Investments
-

 Entertainment4 weeks ago
Entertainment4 weeks agoRed Sox’s Bregman to Become Free Agent; Tigers Commit to Skubal
-

 Science4 weeks ago
Science4 weeks agoNorth Carolina’s Biotech Boom: Billions Invested in Manufacturing
-

 Top Stories4 weeks ago
Top Stories4 weeks agoFormer Mozilla CMO Launches AI-Driven Cannabis Cocktail Brand Fast