Health
Vertex’s Sickle Cell Treatment Faces Delays amid Cell Collection Issues
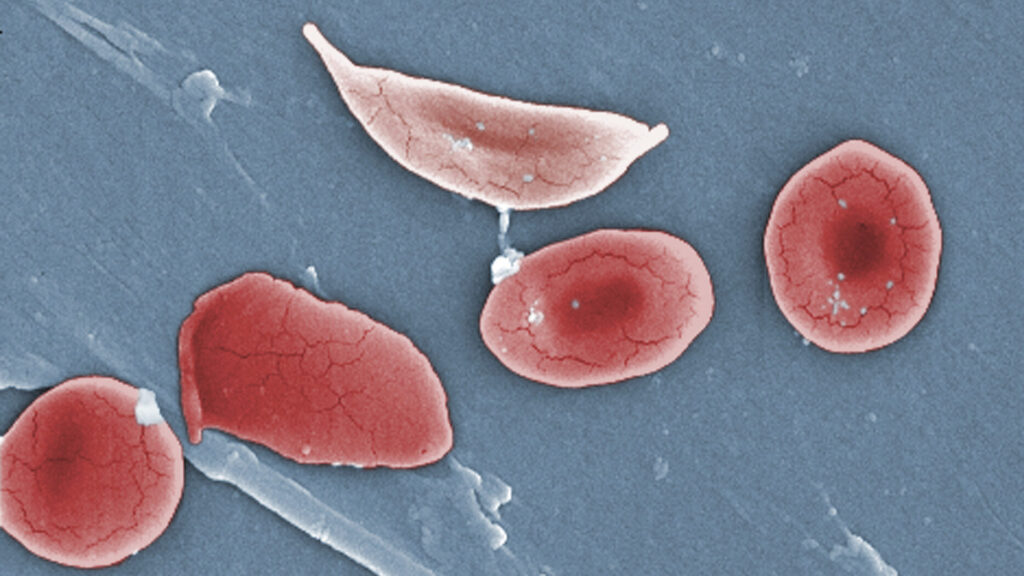
Vertex Pharmaceuticals is encountering significant delays in the rollout of its gene-editing therapy, Casgevy, for sickle cell disease. More than two years after regulatory approval in 2021, only approximately 60 patients across the U.S., Middle East, and Europe have received the treatment. This slow uptake has surprised many specialists in the field who had anticipated a more rapid distribution of the potentially curative therapy.
Challenges in Cell Collection Delay Treatment Expansion
One of the primary hurdles hindering the expansion of Casgevy has been the challenge of collecting enough cells to produce the treatment. Experts at four specialized sickle cell centers have pointed out that the process of gathering the necessary cells has proven to be more complex than initially expected. This issue arises at a critical juncture for Vertex, which is working to establish the market presence of Casgevy while competing against other therapies and preparing for the entry of a significant competitor in the coming year.
The slow progress raises questions about the accessibility of cutting-edge treatments for patients suffering from sickle cell disease, a condition that affects millions worldwide. Vertex’s difficulties highlight the complexities involved in bringing innovative therapies from the laboratory to clinical settings.
Future Implications for Vertex and Competitors
As Vertex navigates these challenges, the company must also contend with the competitive landscape of sickle cell treatments. Rival therapies are gaining traction, and the anticipated launch of a major competitor in 2024 could further complicate the situation for Vertex. The company’s leadership is aware of the pressing need to address the cell collection issue promptly to ensure that Casgevy can reach more patients in a timely manner.
While Vertex executives had previously indicated that the rollout of Casgevy would be gradual, the current pace has been unexpected. As the company seeks to overcome these obstacles, the focus remains on delivering effective treatments to those in need.
In summary, the slow uptake of Casgevy underscores the challenges faced by pharmaceutical companies in the realm of personalized medicine. The journey from innovative research to patient access is often fraught with unforeseen complications, and Vertex’s experience with Casgevy serves as a case study in the complexities of bringing groundbreaking therapies to market.
-

 Science4 months ago
Science4 months agoInventor Achieves Breakthrough with 2 Billion FPS Laser Video
-

 Science2 months ago
Science2 months agoResearchers Launch $1.25M Project for Real-Time Hazard Monitoring in Hawaiʻi
-

 Top Stories4 months ago
Top Stories4 months agoCharlie Sheen’s New Romance: ‘Glowing’ with Younger Partner
-

 Entertainment4 months ago
Entertainment4 months agoDua Lipa Aces GCSE Spanish, Sparks Super Bowl Buzz with Fans
-

 World4 months ago
World4 months agoUK Government Borrowing Hits £20.2 Billion in September Surge
-

 Health4 months ago
Health4 months agoCommunity Unites for 7th Annual Into the Light Walk for Mental Health
-

 Entertainment4 months ago
Entertainment4 months agoMother Fights to Reunite with Children After Kidnapping in New Drama
-

 Entertainment4 months ago
Entertainment4 months agoOlivia Plath Opens Up About Her Marriage Struggles and Divorce
-

 Science3 months ago
Science3 months agoAI Gun Detection System Mistakes Doritos for Weapon, Sparks Outrage
-

 World4 months ago
World4 months agoR&B Icon D’Angelo Dies at 51, Leaving Lasting Legacy
-

 Top Stories4 months ago
Top Stories4 months agoFormer Mozilla CMO Launches AI-Driven Cannabis Cocktail Brand Fast
-

 Health4 months ago
Health4 months agoYouTube Launches New Mental Health Tools for Teen Users








